Medical Device Product Development Journey: A Quick Guide
Table of Contents
- Introduction
- Understanding the Medical Device Development Process
- Medical Device Development Phases
- Common Challenges in Medical Device Development
Introduction
The medical device industry is constantly evolving, driven by innovation and the need for advanced healthcare solutions. Understanding the medical device product development process is crucial for engineers looking to create effective and compliant products. This guide will walk you through the various phases from design to development, highlighting how Chamfr can assist you at each step of the way.
Understanding the Medical Device Development Process
Medical device product development is a structured approach that transforms an idea into a market-ready product. This process is essential not only for ensuring a robust, consistently functioning product but also for meeting efficacy, safety, and regulatory requirements and fulfilling clinical needs. By following a systematic approach, engineers can mitigate risks and improve the chances of successful product launches.
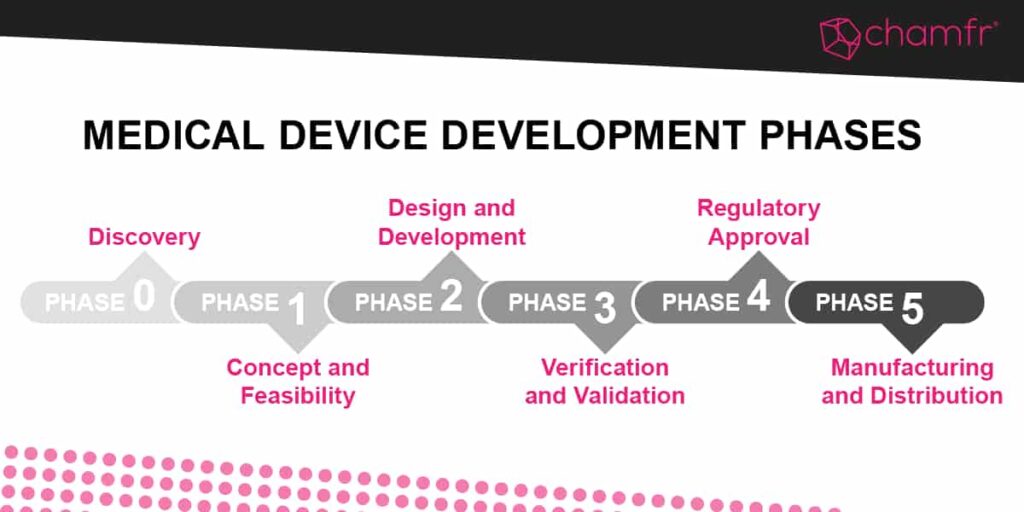
Medical Device Development Phases
- Phase 0: Discovery
The journey starts in the Discovery phase, or Phase 0, where the primary goal is to identify and validate an unmet clinical need. Engineers, product managers, and stakeholders collaborate to define the problem, analyze existing solutions, and explore how a new device could address this gap. Through preliminary research and early-stage market analysis, teams assess the potential impact and technical feasibility of their ideas. With access to Chamfr’s resources, engineers can explore a variety of components, equipment, and tools, gathering the insights needed to lay a strong foundation. This phase emphasizes minimizing risk and establishing a clear vision before advancing to concept development.
- Phase 1: Concept and Feasibility
Building on the foundation laid in the Discovery phase, the journey moves into Concept and Feasibility. Here, engineers refine their initial ideas by conducting detailed market research, brainstorming solutions, and assessing technical requirements. This phase focuses on defining a viable product concept that meets the identified clinical needs. Chamfr’s platform supports engineers in this process, offering easy access to an extensive range of components and access to trusted suppliers.
Chamfr’s platform plays a critical role in this phase, offering engineers rapid access to a wide variety of components in small volumes. This resource enables teams to prototype and iterate quickly, testing concepts without committing to bulk orders. By accessing trusted suppliers and high-quality parts in manageable quantities, Chamfr empowers engineers to minimize waste, control costs, and speed up the iteration process—helping to establish a clear, realistic product concept from the outset and laying a solid foundation for design and development.
- Phase 2: Design and Development
With a viable concept in place, the Design and Development phase begins. Here, engineers move from concept to tangible creation, defining precise product specifications and establishing the initial design framework. This phase encompasses creating prototypes, conducting iterative testing, and refining designs to ensure optimal performance and regulatory compliance. Selecting the right medical device components becomes crucial, as each part must contribute to the product’s efficacy and safety.
Chamfr’s platform is invaluable during this iterative design process, providing access to a wide selection of in-stock components that engineers can quickly source as they test, refine, and adjust their designs. This access helps streamline prototype development, allowing engineers to experiment with various parts without delays, accelerating the entire design cycle.
Chamfr also supports the setup of pilot production lines, offering essential equipment and tools needed to establish R&D labs.
- Phase 3: Verification and Validation
In the Verification and Validation phase, engineers rigorously test and confirm that the device meets all design specifications and user requirements. This phase is divided into two essential components: verification, which ensures the device has been built exactly according to the defined specifications, and validation, which confirms that the product performs as intended in real-world conditions. Together, these steps confirm the device’s reliability, safety, and readiness for regulatory approval.
Chamfr’s platform offers specific advantages during this stage, including access to make-to-order components with lead times of 4 weeks or less. This flexibility allows engineers to source semi-custom components quickly, even if they are not in stock, supporting a streamlined approach to testing without lengthy delays. Additionally, Chamfr provides volume pricing options, giving teams a cost-effective way to source components in higher quantities that align with the demands of extensive verification and validation testing.
By leveraging Chamfr’s suppliers and quick-turnaround make-to-order parts, engineers gain both the documentation needed for regulatory compliance and a reliable supply chain for high-quality components. These resources allow for thorough testing and validation, enabling engineers to progress confidently toward regulatory submission with all necessary documentation and assured product integrity.
- Phase 4: Regulatory Approval
Navigating the regulatory landscape is a significant part of medical device development. Compliance with agencies such as the FDA or CE marking in Europe is essential for market entry. Engineers must prepare and submit extensive documentation to demonstrate the device’s safety and efficacy.
Chamfr’s RFQ (Request for Quote) tool becomes especially valuable at this stage, as it allows engineers to request fully customized components that precisely match the specifications required for regulatory approval. For devices needing specific materials, tolerances, or certifications, Chamfr’s RFQ tool simplifies the search for compliant components by enabling engineers to send a single request to multiple suppliers within Chamfr’s network of 100+ suppliers.
- Phase 5: Manufacturing and Distribution
After regulatory approval, the focus shifts to manufacturing and distribution. This phase involves scaling production while maintaining quality and efficiency. Engineers work closely with manufacturing partners to confirm that all components are assembled accurately, meet quality standards, and are readily available in the required volumes. Effective collaboration with reliable suppliers is essential to ensure a smooth transition from prototype to full-scale production.
Chamfr’s platform is designed to support this critical phase, connecting engineers with a comprehensive supplier directory that includes both low- and high-volume manufacturers. This resource enables teams to find additional manufacturing partners or continue working with trusted suppliers engaged in earlier phases. Whether the production demand is for small initial batches or full-scale manufacturing, Chamfr’s network provides flexibility to scale as needed.
Common Challenges in Medical Device Development
Engineers often face challenges during product development, including rising costs, tight timelines, and complex regulations. One significant hurdle is the difficulty in accessing components early in the development process. Sourcing the right materials and parts can be a time-consuming task, which delays prototyping and testing phases. This complexity can lead to missed opportunities and increased project costs.
To overcome these hurdles, it’s essential to adopt a proactive approach and leverage resources like Chamfr’s platform. By utilizing comprehensive supplier networks and innovative sourcing strategies, engineers can mitigate risks associated with component availability and ensure they have the necessary materials when needed. Chamfr streamlines the sourcing process, allowing engineers to focus on product development and innovation rather than getting bogged down in procurement challenges.
Ready to accelerate your medical device product development?
Create an account today to access components, equipment and tools faster, explore Chamfr’s platform, and connect with suppliers who can support your journey.
Frequently Asked Questions
Medical device product development is a structured process that transforms an idea into a compliant, market-ready medical device through defined phases.
The key Phases include concept and feasibility, design and development, verification and validation, regulatory approval, and manufacturing and distribution.
Chamfr provides access to a wide range of medical device components, expert resources, and supplier connections to streamline the product development process. It’s truly a one-stop shop for medical device product development teams.
Engineers often struggle with finding the right capable suppliers, sourcing components in low volumes with short lead times, which are all needed to be able to iterate quickly throughout the product development journey.
PDP is a structured process guiding each step in medical device development, from identifying needs to completing a regulatory submission. It ensures a standardized approach to quality and compliance throughout product creation.
PLM manages a product from concept through end-of-life, overseeing design, development, manufacturing, and compliance. It helps maintain quality, cost-effectiveness, and regulatory alignment across a product’s entire lifecycle.