Nutek Bravo: When to Consider E-beam Sterilization for Your Device
In this sponsored content post, the team at Nutek Bravo answers common questions about electron beam (e-beam) sterilization. Based in the San Francisco Bay area, Nutek Bravo provides quick-turn electron beam (e-beam) sterilization for batch processing and R&D.
Is e-beam sterilization right for my product?
E-beam sterilization is a great fit for low-density medical devices, human tissue, and lab supplies, as well as biologics and cold-chain products. Nutek Bravo operates two opposing linear accelerators, capable of maintaining very tight dose uniformity ratios (DURs) that many products require. They also have cold-chain equipment for products that require refrigeration or freezing for pre-dwelling, post-dwelling, and/or storage.
E-beam sterilization does not require any post-process dwell as there are no residuals or residue. This makes e-beam very efficient and affordable for complex medical devices such as closed system catheters.
What are the benefits of e-beam sterilization, compared with other methods?
- Greener: Their equipment uses standard electrical power, rather than mining minerals or off-gassing chemicals.
- Mitigated risk: They have a strong, dependable supply chain. Their dual beam system provides redundancy for maximum up-time.
- No quarantine time: Once the product is exposed, it’s ready to ship.
- Box-level processing: You can process an R&D run without simulating a full pallet configuration, adding speed and convenience.
Does Nutek Bravo do both small-lot and large-lot e-beam sterilization?
Yes, they process orders from large-scale batch production to single-lot R&D. Their quality processes enables customers to efficiently bring their products to market.
What is the turn-around time?
The standard turnaround time is 48 hours for batch processing products for market release, with turnaround times of as little as 2 hours available without rush charges. Their dock-high loading zones accommodate trucks with 53-foot trailers to ease shipping and receiving tasks so drivers can be underway as soon as possible. Each pallet of sterilized product comes complete with processing certificates to ensure that products comply with regulations and conform with specifications.
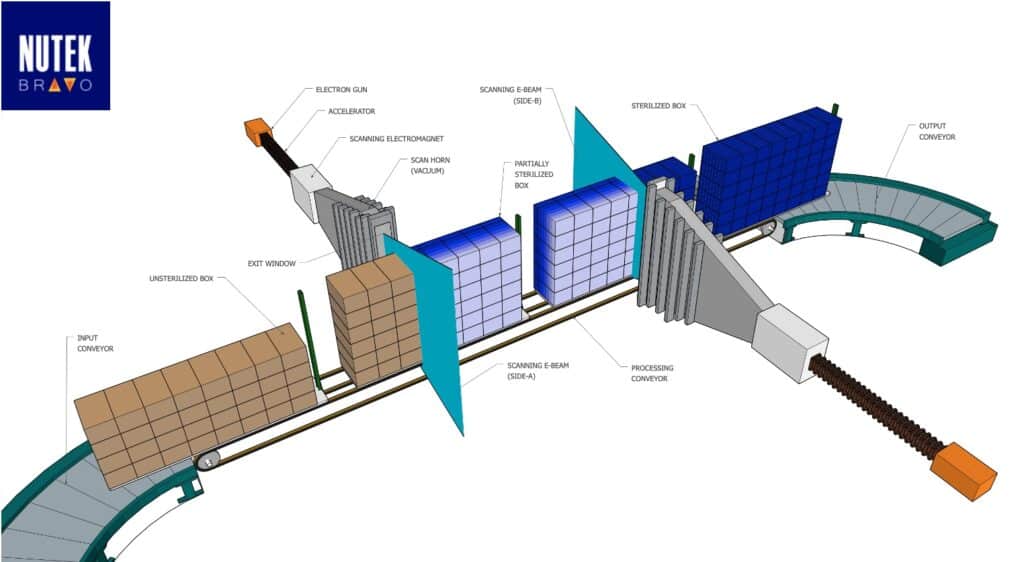
Nutek Bravo’s processing configuration designed to best fit the needs of customers
How does Nutek Bravo validate what dose is needed for sterilization?
They routinely assist their customers with establishing bioburden, substantiating sterilization doses, dose mapping, and sterilization dose audits.
Sterilization process validation can be cumbersome, but they are happy to consult with customers on packaging, configuration, and process decisions.
Typically, they conduct a dose distribution study to locate the product family’s dose extrema. Second, using the identified dose range and its relationship to a reference, the appropriate verification dose is delivered to the samples as part of a verification dose experiment. Finally, they conduct a minimum of three (3) successful dosing experiment replications to adequately map the sterilization processing needs of a product family. This study sets the rules that subsequent batch processing orders must adhere to. When they successfully complete a Dose Map Study, a product family is ready for batch production. Over time, they periodically perform audits of the sterilization dose.
For customers who aren’t quite ready for validation, Nutek Bravo’s engineers and specialists can perform a variety of feasibility runs that target radiation-related properties of any product through its development phase. They offer studies that help manufacturers evaluate material compatibility, electronic tolerance, packaging design, and thermal tolerance. For new product development, they offer a 48-hour turnaround on custom-scope R&D studies. Technical projects and engineering staff help customers to efficiently, effectively advance from product design through routine production.
What is their onboarding process like?
Nutek Bravo’s onboarding process is unmatched in simplicity. Simply navigate to their website complete a New Client Entry Form, and you’re ready to go.
What is the e-beam sterilization process like?
Experience the irradiation journey from the perspective of the product in this mesmerizing short video.
If you have questions about whether e-beam sterilization is right for your product, contact them at sales@nutekbravo.com or call (510) 900-9670.
Nutek Bravo, a Chamfr Service Partner, is an ISO-certified, FDA-registered contract sterilization partner providing quick-turn electron beam (e-beam) sterilization for batch processing and R&D. Check out our Service Partner page for more partners to help you execute on your design & development project.