
If you’re making single-use devices that require standard push-pull connectors like Lemo, Fischer, or ODU, this blog is for you.

If you’re making single-use devices that require standard push-pull connectors like Lemo, Fischer, or ODU, this blog is for you.

Connectors are essential in various medical devices. They demand advanced designs but also the utilization of premium materials and intricate manufacturing and validation processes.

With recent advancements in single-use (disposable) medical technologies, OEMs must achieve a single-use medical connector design that’s cost-effective, scalable, and reliable.
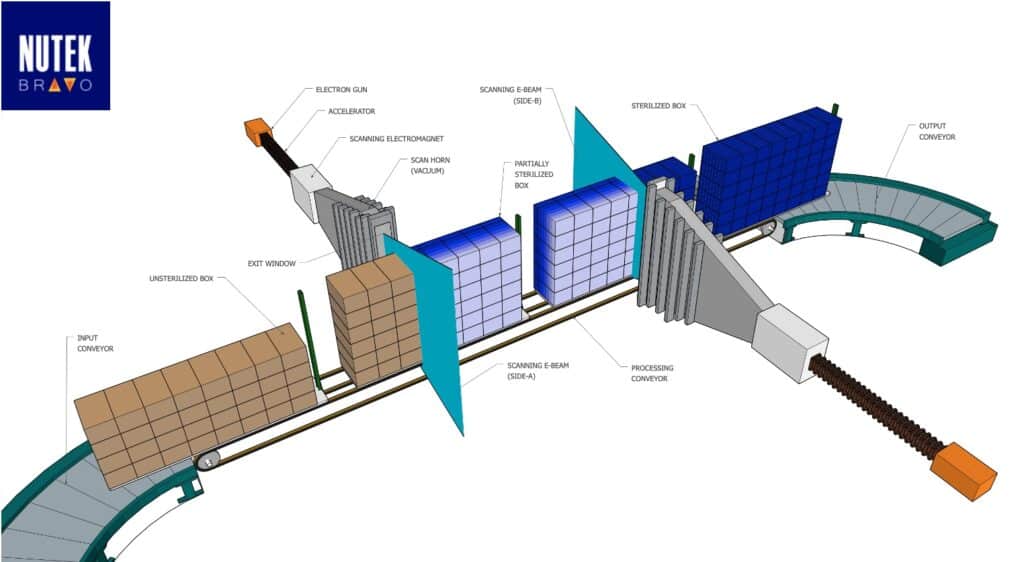
With a team of well versed engineers, Nutek Bravo isn’t just a supplier but a partner when it comes to contract sterilization. They work closely with their customers to ensure that both the science behind the work and service their customers receive are of the utmost quality.

Effectively applying software and artificial intelligence (A.I.) based vision tools to quality control, decision making, process improvement and automation. Reduce your inspection cost while making it more reliable.

In this blog titled “Plastic Injection Molding Process Guide”, produced by the subject matter experts at BMP Medical, we offer educational information that will help you build better injection molded medical devices and components.